-
Products
- Local Securities
- China Connect
- Grade Based MarginNEW
- Stock Borrowing & Lending
- IPO
- Stock Options
- Foreign Stocks
- Unit Trust
- Local Futures
- Foreign Futures
- Forex
- Bullion
- Insurance Services
- Bond
- Monthly Investment Plan
- Mortgage
- Other Services
- Surplus Cash Facility
- Phillip Premier
- Latest Insurance Promotion<
- ETF
- Capital Management
- Research
- Market Info
- Education Center
- Phillip Apps
- Customer Service
- About Us
-
Surplus Cash Facility
Weekly Specials
CR PHARMA (3320.HK) - Develop Segments Cooperation, Improve Business Synergy
Tuesday, November 26, 2019  9784
9784
CR PHARMA(3320)
| Recommendation | BUY |
| Price on Recommendation Date | $6.740 |
| Target Price | $11.220 |
Weekly Special - 3306 JNBY Design Limited
Subsidiaries Maintained Growth in 3Q2019
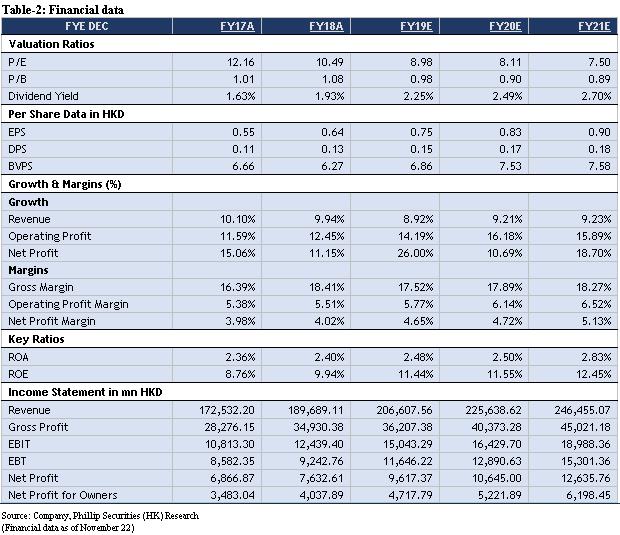
For the nine months ended September 30, 2019, the operating revenue of CR Pharmaceutical Holdings, a wholly-owned subsidiary of the company, was RMB 135.022 billion (3Q2018: RMB 116.795 billion), representing an increase of 15.6% YoY; the net profit attributable to shareholders was RMB 2.465 billion (3Q2018: RMB 1.804 billion), a YoY increase of 36.6%.
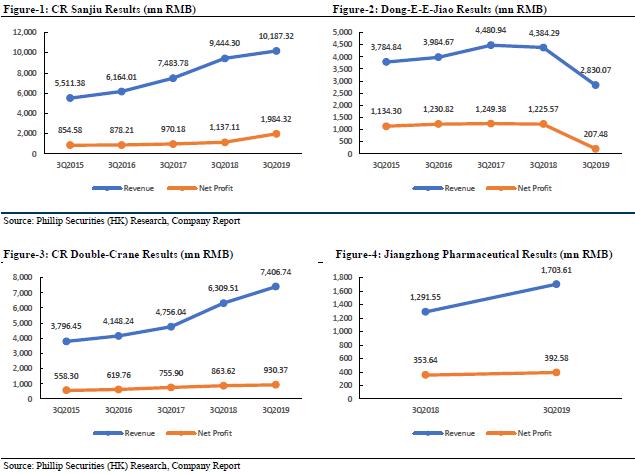
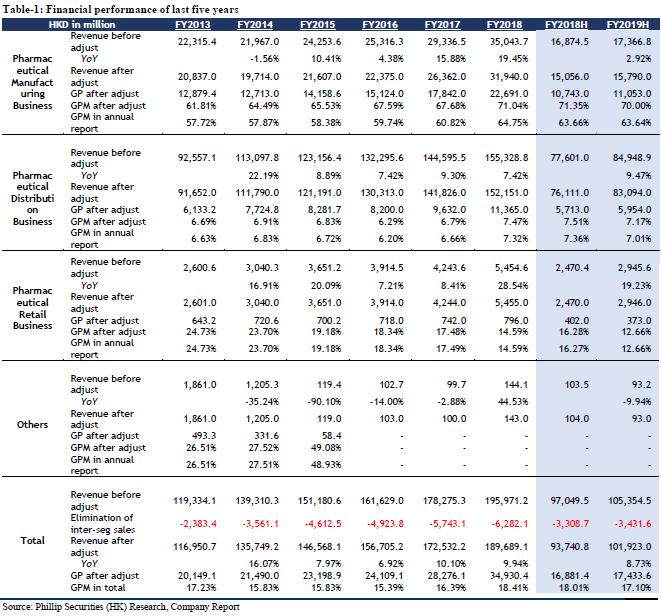
For the nine months ended September 30, 2019, the revenue of Dong-E-E-Jiao, a subsidiary of the company, was RMB 2.83 billion (3Q2018: RMB 4.394 billion), a YoY decrease of 35.59%; the net profit was RMB 207 million (3Q2018: RMB 1.226 billion), a YoY decrease of 83.12%. The revenue of CR Sanjiu, a subsidiary of the company, was RMB 10.187 billion (3Q2018: RMB 9.444 billion), a YoY increase of 7.87%; the net profit was RMB 1.984 billion (3Q2018: RMB 1.137 billion), a YoY increase of 74.49%; based on preliminary assessment by the management of CR Sanjiu, the unaudited net profit attributable to the shareholders of CR Sanjiu for the year ended 31 December 2019 are estimated to be RMB 2.11 to 2.25 billion (FY2018: RMB 1.432 billion), an expected increase of 47.34% to 57.11% YoY; the significant increase in the net profit attributable to the shareholders is primarily attributable to the completion of disposal of its 82.89% equity interest in Shenzhen Sanjiu Hospital Co., Ltd. in January 2019, resulting in a net gain (after tax) of approximately RMB 680 million to CR Sanjiu. The revenue of CR Double-Crane, a subsidiary of the company, was RMB 7.407 billion (3Q2018: RMB 6.31 billion), a YoY increase of 17.39%; the net profit was RMB 930 million (3Q2018: RMB 864 million), a YoY increase of 7.64%. The revenue of Jiangzhong Pharmaceutical, a subsidiary of the company, was RMB 1.704 billion (3Q2018: RMB 1.292 billion), a YoY increase of 31.89%; the net profit was RMB 393 million (3Q2018: RMB 354 million), a YoY increase of 11.02%. In general, apart from Dong-E-E-Jiao, the performance of the company's subsidiaries has maintained steady growth, and with the gradual progress of Dong-E-E-Jiao, we are still optimistic about the company's performance as the industry leader in the future.
Continue to Increase and Optimize Product portfolio in the Pharmaceutical Sector
Recently, the company pointed out that it will use the channel advantage in the pharmaceutical sector to continuously increase and optimize its product portfolio in "CICC Forum 2019". The manufacturing business of the company encompasses the research and development, manufacturing and sale of pharmaceutical products. The company manufactured more than 540 products in 1H2019, of which morethan 300 were included in NRDL. The products comprise chemical drugs, Chinese medicines and biopharmaceutical drugs as well as nutritional and healthcare products, covering a wide range of therapeutic areas including cardiovascular, alimentary tract and metabolism, large-volume IV infusion, pediatrics, respiratory system etc.. The company had approximately 200 R&D projects in the pipeline, including 45 projects in the pipeline on innovative drugs. In addition, on October 3, 2019, NIP292, an innovative drug developed by the China Pharmaceutical Research and Development Center directly under the company's research and development platform, was approved by the US Food and Drug Administration (FDA) clinical trial and conducted a phase I clinical trial in the United States. NIP292 is primary treatment of pulmonary fibrosis (IPF), it is a new small molecule drug with multiple functions such as anti-inflammatory, anti-fibrosis, dilation of blood vessels, and repair of vascular endothelial injury. In addition to IPF, NIP292 has great potential for the treatment of autoimmune diseases, other fibrotic diseases, and malignant tumors. On October 23, CR Sanjiu, a subsidiary of the company, and Japan's Takeda Consumer Health Co., Ltd. signed an ALINAMIN product cooperation agreement in Beijing. CR Sanjiu will be responsible for the commercialization and sales of ALINAMIN in the Chinese market, and the two sides will also reach a consensus on other product portfolios and cross-border e-commerce business in the future. The company continues to improve R&D capability, enrich pipeline and access to products, and forge advantages of brand clustering, which is believed to benefit future development.
Market Share of the Circulation Sector is Expected to Further Increase
As at the end of 1H2019, distribution network of the company covered 28 provinces, reached 141 cities in total, serving over 100,000 downstream customers, including 6,862 Class II & III hospitals, and 53,640 primary medical institutions competitiveness in the Eastern China. The company continuously enhanced efficiency of the integrated and modernized intelligent logistics system, as at the end of 1H2019, the company operated 185 logistics centers in total. The company enhanced capability in providing value-added services to downstream customers, provided Hospital Logistic Intelligence (HLI) services to over 300 hospitals, and commenced Network Hospital Logistics Intelligence (NHLI) projects. In 1H2019, the company operated 842 retail pharmacies, of which 150 are DTP pharmacies covering 76 cities nationwide. The company continues to deepen the layout of pharmaceutical circulation sector, and enhances strength of the company through endogenous expansion and outreach acquisition, its market share is expected to further increase.
Maintain "BUY" Rating
We maintain the expected EPS of HKD 0.75/0.83/0.90. The target price was HKD 11.22, corresponding to FY19/FY20/FY21 14.95x/13.51x/12.48x PE, which was +66.51% higher than the current price (HKD 6.74 as of November 22, 2019), maintaining a “BUY” rating.
Risk
Industry policy risk; M&A fails expectations.
Financials
This report is produced and is being distributed in Hong Kong by Phillip Securities Group with the Securities and Futures Commission (“SFC”) licence under Phillip Securities (HK) LTD and/ or Phillip Commodities (HK) LTD (“Phillip”). Information contained herein is based on sources that Phillip believed to be accurate. Phillip does not bear responsibility for any loss occasioned by reliance placed upon the contents hereof. The information is for informative purposes only and is not intended to or create/induce the creation of any binding legal relations. The information provided do not constitute investment advice, solicitation, purchase or sell any investment product(s). Investments are subject to investment risks including possible loss of the principal amount invested. You should refer to your Financial Advisor for investment advice based on your investment experience, financial situation, any of your particular needs and risk preference. For details of different product's risks, please visit the Risk Disclosures Statement on http://www.phillip.com.hk. Phillip (or employees) may have positions/ interests in relevant investment products. Phillip (or one of its affiliates) may from time to time provide services for, or solicit services or other business from, any company mentioned in this report. The above information is owned by Phillip and protected by copyright and intellectual property Laws. It may not be reproduced, distributed or published for any purpose without prior written consent from Phillip.
Top of Page
|
Please contact your account executive or call us now. Research Department Tel : (852) 2277 6846 Fax : (852) 2277 6565 Email : businessenquiry@phillip.com.hk Enquiry & Support Branches The Complaint Procedures |
About Us Phillip Securities Group Join Us Phillip Network Phillip Post Phillip Channel Latest Promotion |
E-Check Login |
Investor Notes Free Subscribe |

|






