-
Products
- Local Securities
- China Connect
- Grade Based Margin
- Stock Borrowing & Lending
- IPO
- Stock Options
- Foreign Stocks
- Unit Trust
- Local Futures
- Foreign Futures
- Forex
- Bullion
- Insurance Services
- Bond
- Monthly Investment Plan
- Mortgage
- Other Services
- Surplus Cash Facility
- Phillip Premier
- Latest Insurance Promotion<
- ETF
- Smart Minor (Joint) Account
- Capital Management
- Research
- Market Info
- Education Center
- Phillip Apps
- Customer Service
- About Us
-
Surplus Cash Facility
Research Report
Fosun Pharma (2196.HK) - Overseas Acquisitions to Expand International Business
Tuesday, August 16, 2016  11697
11697
Fosun Pharma(2196)
| Recommendation | Buy |
| Price on Recommendation Date | $19.000 |
| Target Price | $23.600 |
Weekly Special - 175 Geely
Overseas Acquisitions to Expand International Business
Fosun Pharma is going to acquire approximately 86.08% stake in Gland Pharma Limited of India for no more than $ 1.26 billion through its subsidiaries, including the no more than $50 million as the most likely amount that the acquirer has paid in accordance with the sales of Enoxaparin on the American market.
The company plans to forge Gland into an international pharmaceutical manufacturing and registration platform. Gland is the first injectables manufacturer in India that has got approval from the FDA of America and from GMP in Europe and America. Its revenue comes therefore mainly from America and Europe. Currently, it is providing manufacturing services of injectable drugs to large pharmaceutical companies around the world through joint development and import licensing. The business advantages and experience of Gland will therefore be conducive to Fosun in its way forward to the international market. Indian market has the advantage of conformance with international standards in drug development, clinical tests and manufacturing process, among which Gland is a leading company.
Synergy Effect Seems Significant
Firstly, in terms of product, Gland has a very diversified product line with a total of approximately 120 products different in type and form, involving mainly sterile injectables like heparin sodium and Enoxaparin sodium. With its whole industry chain of heparin, Fosun Wanbang, once granted approval from the international market, will be able to supply Gland with superior heparin raw materials. The FDA approved production line of Yaopharma in Chongqing could also complement the production capacities of Gland effectively. The total sales of Enoxaparin injection around the world has amounted to $3 billion (1 billion of which achieved in America). As Gland Pharma Limited Enoxaparin has been launched in Europe and the chances for it to be approved in America are also high, it is expected to have a huge space for growth.
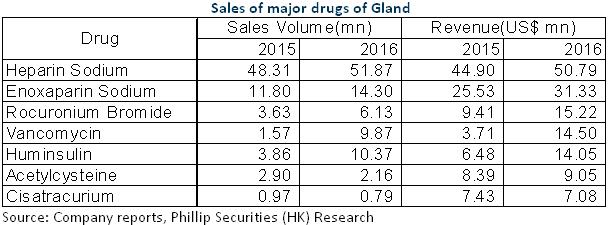
In addition, the registration team of Gland Pharma Limited has 300 to 400 employees and has obtained the authorization to produce and sell products in over 70 countries and regions beyond India. This will support the registration of Fosun drugs (like biological drugs, insulin and mAb) in the world, which are therefore expected to speed up the entry into American and Indian markets. In terms of generic drugs, if the products could obtain approval from the FDA of America, they will not have to go through consistency evaluation in mainland China. That means the company is expected to enter the market earlier, thus seizing more market shares. Meanwhile, the drugs of Gland Pharma are also expected to enter the domestic market via Fosun.
Steady Growth Supported by a Whole Industrial Chain and a Global Layout
This acquisition will further enhance the competitive advantages of Fosun Pharma in drug development and production, besides enriching its product line. Combined with the layout in such sectors as medical services, medical devices and pharmaceutical distribution, the company has become one of the few companies in the industry with a whole industry chain. Meanwhile, the company has expanded its layout to such trans-regional markets known for the space for growth as China, America and India, thus making a steady growth more anticipated in the future. We give an estimation of 16x EPS in 2016, and a target price of HKD 23.6, with the "Buy" rating upgraded. (Closing price as at 11 Aug 2016)
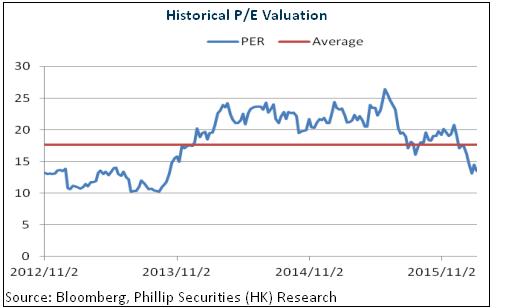
Risks
Price drop;
Acquisition and integration falls short expectation;
Geopolitical risks in the expansion of overseas business.
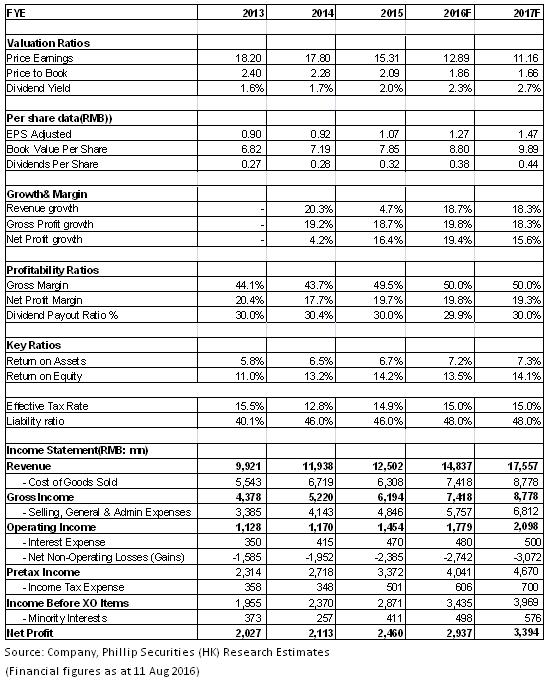
Financials
This report is produced and is being distributed in Hong Kong by Phillip Securities Group with the Securities and Futures Commission (“SFC”) licence under Phillip Securities (HK) LTD and/ or Phillip Commodities (HK) LTD (“Phillip”). Information contained herein is based on sources that Phillip believed to be accurate. Phillip does not bear responsibility for any loss occasioned by reliance placed upon the contents hereof. The information is for informative purposes only and is not intended to or create/induce the creation of any binding legal relations. The information provided do not constitute investment advice, solicitation, purchase or sell any investment product(s). Investments are subject to investment risks including possible loss of the principal amount invested. You should refer to your Financial Advisor for investment advice based on your investment experience, financial situation, any of your particular needs and risk preference. For details of different product's risks, please visit the Risk Disclosures Statement on http://www.phillip.com.hk. Phillip (or employees) may have positions/ interests in relevant investment products. Phillip (or one of its affiliates) may from time to time provide services for, or solicit services or other business from, any company mentioned in this report. The above information is owned by Phillip and protected by copyright and intellectual property Laws. It may not be reproduced, distributed or published for any purpose without prior written consent from Phillip.
Top of Page
|
Please contact your account executive or call us now. Research Department Tel : (852) 2277 6846 Fax : (852) 2277 6565 Email : businessenquiry@phillip.com.hk Enquiry & Support Branches The Complaint Procedures |
About Us Phillip Securities Group Join Us Phillip Network Phillip Post Phillip Channel Latest Promotion 新闻稿 |
E-Check Login |
Investor Notes Free Subscribe |

|




